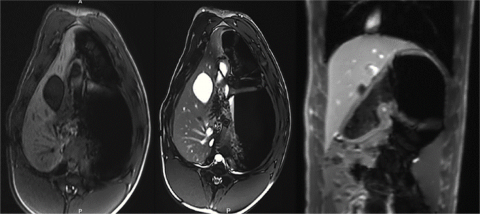
While global cancer mortality is generally decreasing, the incidence of hepatocellular carcinoma (HCC) is projected to increase given the growing prevalence of chronic liver diseases that increase the risk for carcinogenesis. The current diagnosis of liver cirrhosis is invasive tissue biopsy, and those at-risk undergo an HCC surveillance program of ultrasound imaging to survey tumor development. Such screening programs center on static radiologic imaging snapshots prescribed at arbitrary intervals based on empiric histologic cirrhosis staging schemes and observational tumor developmental data that neither reflect the transition between normal and diseased states nor biologically relevant disease thresholds. In contrast, magnetic resonance imaging (MRI) and magnetic resonance elastography (MRE) may provide a more foundational portrayal of early stages of liver disease and liver oncogenesis that could result in early diagnosis when curative therapies could be employed. In addition, MRI/MRE-correlated systemic molecular biomarkers indicative of specific disease stages also have potential to serve as important non-invasive diagnostic tools. In this study, we developed a clinically translatable MRI/MRE liver imaging protocol in a clinically relevant large animal platform, the Oncopig Cancer Model (OCM). The OCM is a novel transgenic swine model that recapitulates human HCC through development of site and cell specific tumors after Cre recombinase induced expression of heterozygous KRASG12D and TP53R167H transgenes. A clinical imaging workflow consisting of respiratory gated acquisitions was developed with the Siemens 3 T Prisma MRI scanner. This novel imaging protocol successfully provides high quality, high resolution MRI/MRE images for clinical interpretation. In the future, integration of MRI/MRE, histology, and molecular measures in Oncopigs presenting with liver cirrhosis and HCC may be employed to investigate biomarkers that may define normal versus diseased liver, and may serve as a new approach to identify optimal time points for initiation of disease and treatment for clinical translation. This study is currently funded by Army W81XWH-16-1-0335.