Introduction: The orphan nuclear receptor (NR) family member 4A sub-type 1 (NR4A1/TR3) exhibits genomic activity as a transcription factor in the cell nucleus and nongenomic activity as a cytosolic mediator of apoptosis. Their multiple small molecule agonists, through nuclear hormone receptor activation, has been shown to suppress cancer invasion, angiogenesis, tumor progression, and metastasis but lack of aqueous solubility, dose-limiting toxicities and lack of objective responses limit use of available RXR-selective retinoid for efficient treatment. Variations in functional entities on bis(indolyl)methane backbone leading to better interaction with hinge region of the ligand and amine residues C432, I268 from RXR protein might provide better selectivity and in turn low dose-limiting toxicities. Once synthesized, an animal model close to human system, onco-pig, would help in evaluating drug kinetics, clearance and affectivity.
Materials a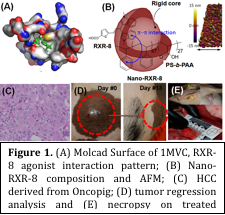 nd Methods: Structure-based drug discovery involving computational modeling studies was used for deciding the best-fit structure of ligand against Molcad Surface of 1MVC, RXR agonist conformation crystal structure, for transcriptional activation of NR4A1/TR3. The optimium chemistry of RXR-8 (Figure 1; Bis(indolyl)methane derivative) was ably synthesized in laboratory with ~81% yield by conjugating cinnamaldehyde with 1-methylindole in controlled condition and characterized by mass and NMR analysis. RXR-8 was loaded in amphiphilic polymer generated rigid core micelles (Nano-RXR-8) and dialyzed against buffer of pH 7.4 to remove free molecules. Integrity of Nano-RXR-8 was characterized by DLS, Zeta potential, UV-vis, SEM, TEM and AFM imaging methods and found to be stable. In vitro cytotoxicity assays were performed for RXR-8 and Nano-RXR-8 in cancer cells of pig origin including HepatoCre_63-4 and HepatoCre_0327. Onco-pigs were developed by encoding Cre recombinase inducible porcine transgenes encoding KRASG12D and TP53R167H. A tumor regression analysis was performed in onco-pigs after intra-tumoral treatment and followed by ultrasound imaging. Gene expression analysis was performed on RNA samples extracted from tumor and liver tissues of the treated onco-pigs followed by histopathlogy. A clinical pathology was performed on blood serum and urine samples collected from treated onco-pigs.
nd Methods: Structure-based drug discovery involving computational modeling studies was used for deciding the best-fit structure of ligand against Molcad Surface of 1MVC, RXR agonist conformation crystal structure, for transcriptional activation of NR4A1/TR3. The optimium chemistry of RXR-8 (Figure 1; Bis(indolyl)methane derivative) was ably synthesized in laboratory with ~81% yield by conjugating cinnamaldehyde with 1-methylindole in controlled condition and characterized by mass and NMR analysis. RXR-8 was loaded in amphiphilic polymer generated rigid core micelles (Nano-RXR-8) and dialyzed against buffer of pH 7.4 to remove free molecules. Integrity of Nano-RXR-8 was characterized by DLS, Zeta potential, UV-vis, SEM, TEM and AFM imaging methods and found to be stable. In vitro cytotoxicity assays were performed for RXR-8 and Nano-RXR-8 in cancer cells of pig origin including HepatoCre_63-4 and HepatoCre_0327. Onco-pigs were developed by encoding Cre recombinase inducible porcine transgenes encoding KRASG12D and TP53R167H. A tumor regression analysis was performed in onco-pigs after intra-tumoral treatment and followed by ultrasound imaging. Gene expression analysis was performed on RNA samples extracted from tumor and liver tissues of the treated onco-pigs followed by histopathlogy. A clinical pathology was performed on blood serum and urine samples collected from treated onco-pigs.
Results and Discussion: Nano-RXR-8 were 2-4 fold more effective and 8 fold more selective against HCC cells (HepatoCre_63-4) compared to normal hepatic cell line with respect to small molecule RXR-8 at 48 and 72h time points. Safety profile of Nano-RXR-8, along with RXR-8 and nanoparticle alone was studied by clinical pathology and found to be affecting no clinical parameter to any significant level. Tumor regression analysis revealed a 20 fold decrease in tumor size of onco-pig treated by RXR-8 and ~40 fold by Nano-RXR-8 compared to untreated onco-pigs. Transcriptional alteration of genes involved in phase-I and phase-II drug metabolism, phase-III transport and nuclear receptors in liver measured by qPCR showed better upregulation of orphan nuclear receptors (regulators) after treatment with Nano-RXR-8 compared to RXR-8 in treated onco-pigs compared to untreated. Histopathological analysis of tumor sections from treated onco-pigs revealed the occurrence of apoptotic lesions with minimal cellular swelling in liver sections (Figure 1).
Conclusions: Nano-RXR-8 were developed as efficient RXR regulators as identified by structure based drug discovery, synthesized by efficient chemical synthesis and nano formulation preparation with no significant effect on clinical parameters of onco-pigs and high tumor regression ability.
